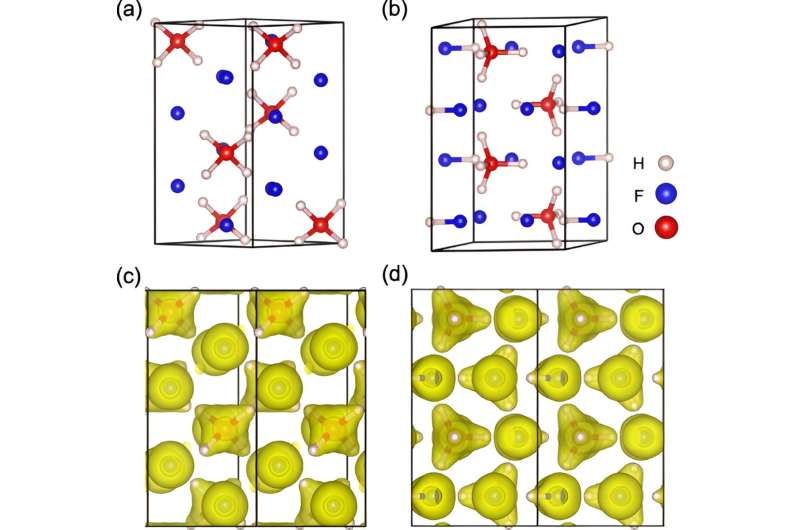
Crystal structures and electronic localization function of H4OF2 and H4OF2·HF. Credit: Physical review B (2024). DOI: 10.1103/PhysRevB.109.174102
Skoltech scientists and their Chinese colleagues have determined the conditions that enable the existence of a very special ion. Called aquodiium, it can be thought of as an ordinary neutral water molecule with two extra protons stuck to it, resulting in a net double positive charge.
The team suggests that the ion may be stable in the interior of the ice giants Uranus and Neptune, and if so, it should play a role in the mechanism that creates these planets’ unusual magnetic fields. The study was published in Physical review B
Strange magnetism
The magnetic fields of Uranus and Neptune are not as well understood as those of Jupiter and Saturn – or our planet, for that matter.
In the Earth’s interior, the circulation of the electrically conductive liquid iron-nickel alloy produces magnetism. Deep inside Jupiter and Saturn, hydrogen is thought to be compressed into a metallic state and create magnetic fields in the same way.
In contrast, the magnetic fields of Uranus and Neptune are assumed to derive from the circulation of ionically conducting media, where the constituent ions are themselves charge carriers, rather than simply a support structure that enables the flow of electrons.
If planetary scientists knew exactly what ions are involved and in what sizes, perhaps they could understand why the magnetospheres of the ice giants are so strange: misaligned with the direction of rotation of the planets and displaced from their physical centers.
Skoltech professor Artem R. Oganov, who co-authored the paper, explains how ionic and electronic conductivity are different and where the newly predicted ion fits into this: “The hydrogen surrounding Jupiter’s rocky core under those conditions is a liquid metal : can flow , the way molten iron flows in the Earth’s interior and its electrical conductivity is due to the free electrons shared by all the hydrogen atoms pressed together.
“On Uranus, we think that the hydrogen ions themselves—that is, the protons—are free charge carriers. Not necessarily as independent H+ ions, but probably in the form of hydronium H3O+ammonium NH4+, and a variety of other ions. Our study adds another possibility, H4O2+ ion, which is extremely interesting from a chemical point of view.”
Missing connection
In chemistry, there is the notion of sp3 hybridization, which refers to the way electron orbitals combine with each other and constitutes something of a natural template for creating reliable molecules and ions. Under sp3 hybridization, the nucleus of an atom – e.g. carbon, nitrogen, or oxygen—occupies the central point of an imaginary tetrahedron.
Each of the four vertices houses either one valence electron or two unpaired electrons that are not available to form bonds with other atoms. The simplest example would be a carbon atom with four unpaired electrons in the four vertices – add four hydrogen atoms and you get a methane molecule: CH4.
For an oxygen atom, which has two pairs of lone electrons in the outermost shell, along with two valence electrons, the sp.3 hybridization will mean that only two of the vertices can hold a covalent hydrogen bond, with the other two occupied by electron pairs, which give H2Oh, water.
If you attach a hydrogen ion (a proton) to one of the electron pairs, you get a hydronium ion H3O+and that’s actually what you get in an acid solution, because acids donate H protons+ in solution and the lone protons are immediately attracted to the electron pairs.
Pressure and acid
“But the question was: Can you add another proton to the hydronium ion to fill the missing part? Such a configuration under normal conditions is energetically very unfavorable, but our calculations show that there are two things that can make it happen,” he says. Professor Xiao Dong from China’s Nankai University, whose original idea underlies this research.
“First, very high pressure forces matter to reduce its volume, and sharing a previously unused electron pair of oxygen with a hydrogen ion (proton) is a good way to do this: as a covalent bond with hydrogen , except for the two electrons in the pair. Second, you need a lot of protons available, and that means an acidic environment, because acids donate protons.
The team used advanced computational tools to predict what happens to hydrofluoric acid and water under extreme conditions. Result: Given a pressure of about 1.5 million atmospheres and a temperature of about 3,000 degrees Celsius, aqueodium H separates well4O2+ ions appear in the simulation.
The scientists believe that their newly discovered ion should play an important role in the behavior and properties of water-based media, especially those under pressure and containing acid.
This roughly corresponds to the conditions of Uranus and Neptune, where an extremely deep water ocean produces extremely high pressures, and some acid can also be expected. If so, aquadium ions would form and, by participating in ocean circulation, would contribute to these planets’ magnetic fields and other properties in ways different from other ions.
Perhaps, aquodium can also form as yet unknown minerals under those extreme conditions.
More information:
ingyu Hou et al, H4O2+ pressure stabilized ion, Physical review B (2024). DOI: 10.1103/PhysRevB.109.174102
Provided by Skolkovo Institute of Science and Technology
citation: A strange molecule may be hiding inside Uranus and Neptune, affecting their magnetic fields (2024, May 31) Retrieved June 1, 2024 from https://phys.org/news/2024-05-outlandish-molecule- lurking-uranus-neptune .html
This document is subject to copyright. Except for any fair agreement for study or private research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
#strange #molecule #hiding #Uranus #Neptune #affecting #magnetic #fields
Image Source : phys.org